摘要
Lead is a metal that has toxic effects on the developing nervous system. However, the mechanisms underlying lead-induced neurotoxicity are not well understood. Non-coding RNAs (ncRNAs) play an important role in epigenetic regulation, but few studies have examined the function of ncRNAs in lead-induced neurotoxicity. We addressed this in the present study by evaluating the functions of a long non-coding RNA (named lncRpa) and a circular RNA (named circRar1) in a mouse model of leadinduced neurotoxicity. High-throughput RNA sequencing showed that both lncRpa and circRar1 promoted neuronal apoptosis. We also found that lncRpa and circRar1 induced the upregulation of apoptosis-associated factors caspase8 and p38 at the mRNA and protein levels via modulation of their common target microRNA miR-671. This is the first report of a regulatory interaction among a lncRNA, circRNA, and miRNA mediating neuronal apoptosis in response to lead toxicity.
关键词:CircRNA、LncRNA、MiRNA、Cell apoptosis、Lead Neurotoxicity
研究背景
铅作为一种神经发育性强烈毒药,会导致神经性坏死。然而,关于铅致神经中毒的机理目前并没有很好的解释。非编码RNA(ncRNAs)在表观遗传调控领扮演者重要的角色,但是,ncRNA参与铅致神经中毒的功能研究却很少,本研究突出了lncRNAs、circRNAs和miRNAs的相互作用,旨在解释ncRNAs在铅中毒神经细胞凋亡过程中的调控机制。
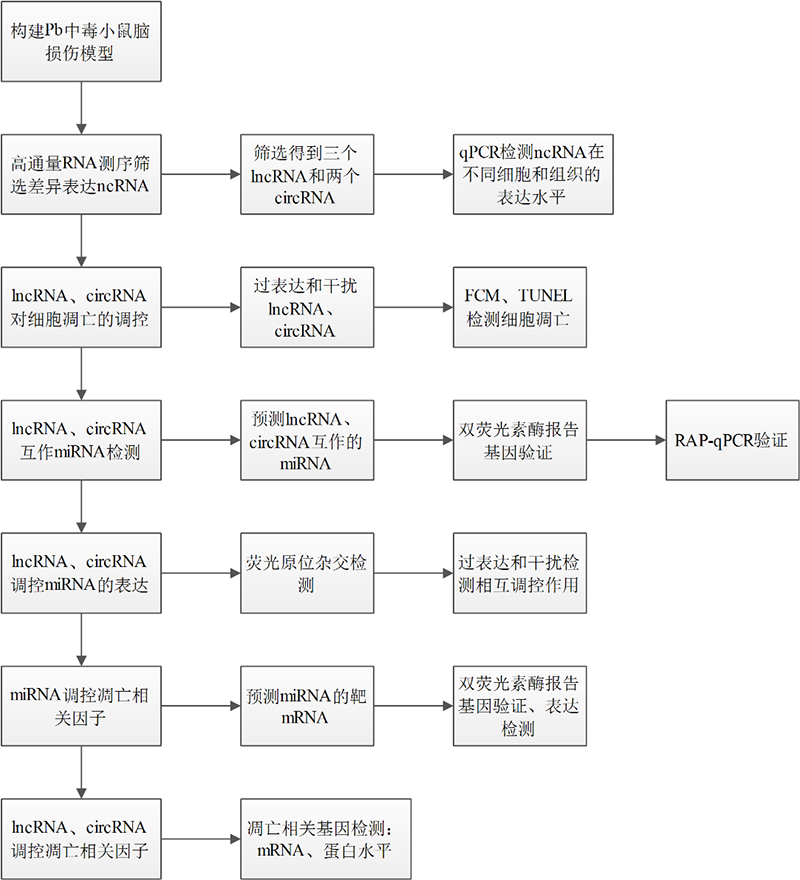
方法流程
部分研究结果
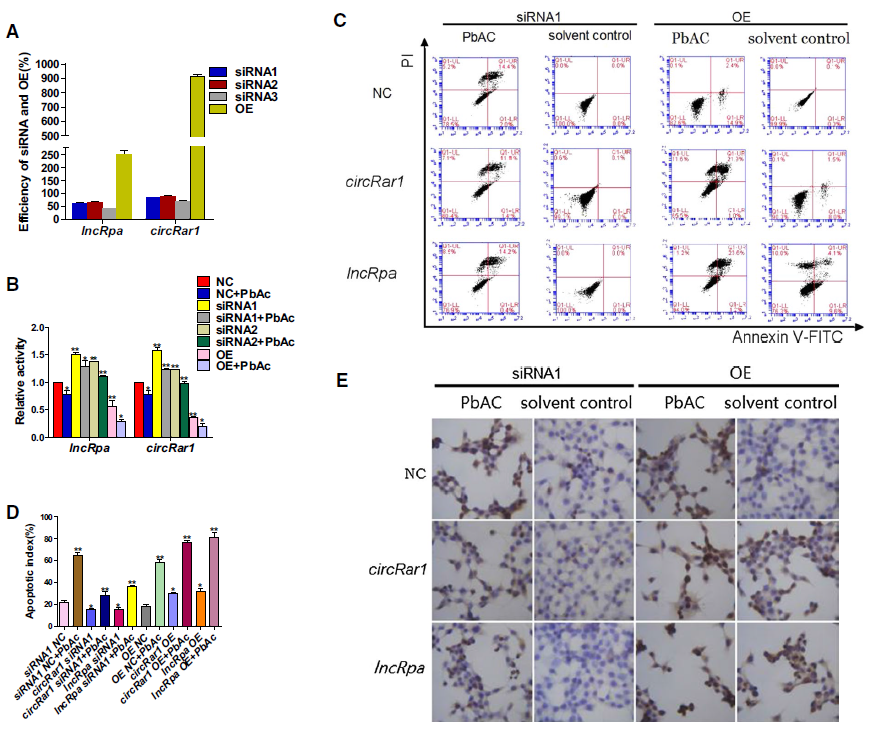
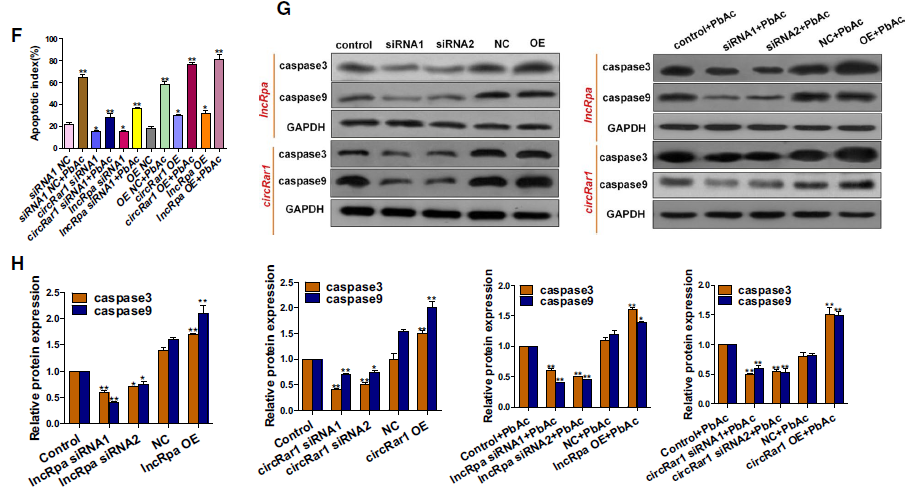
1. LncRpa 和 circRar1促进细胞凋亡
分别过表达和干扰lncRpa、circRar1,检测过表达和RNA干扰效率,利用FCM和TUNEL进行凋亡检测,同时对凋亡相关蛋白进行表达检测。
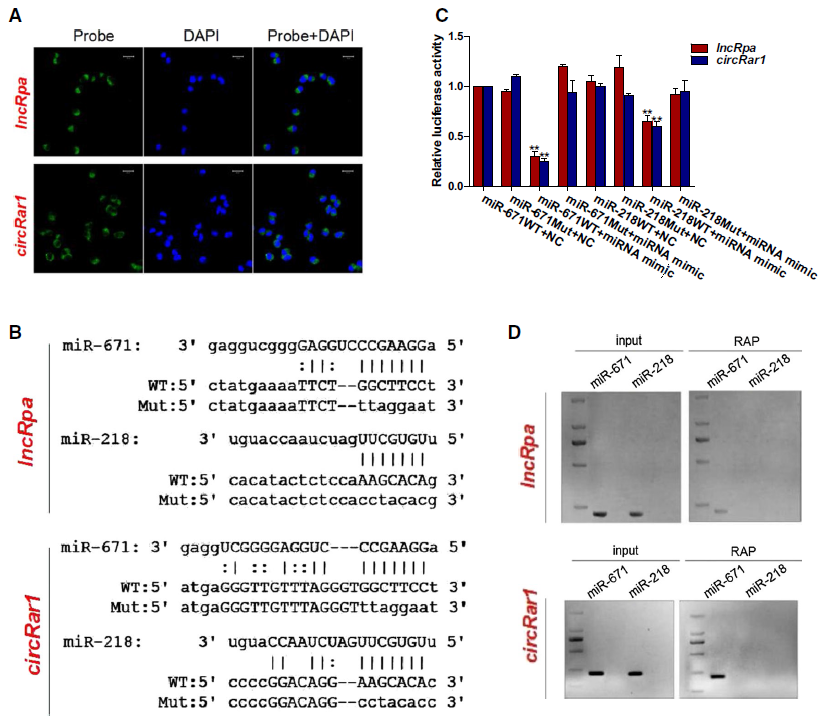
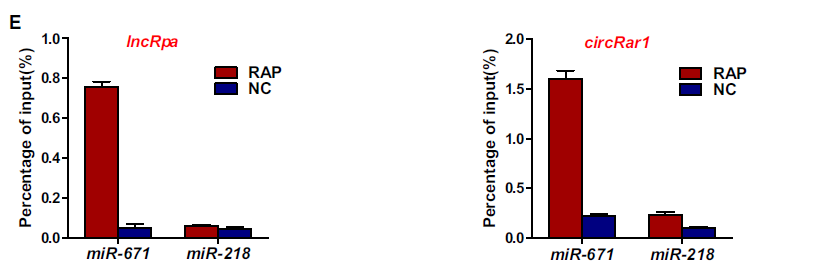
2. LncRpa、circRar1直接互作miR‑671
首先利用数据库预测lncRpa、circRar1共同互作的miRNA,荧光原位杂交(FISH)进行lncRpa、circRar1定位,再开展荧光素酶报告基因和RAP-qPCR进行验证。结果显示,lncRpa、circRar1能够与miR‑671直接互作。
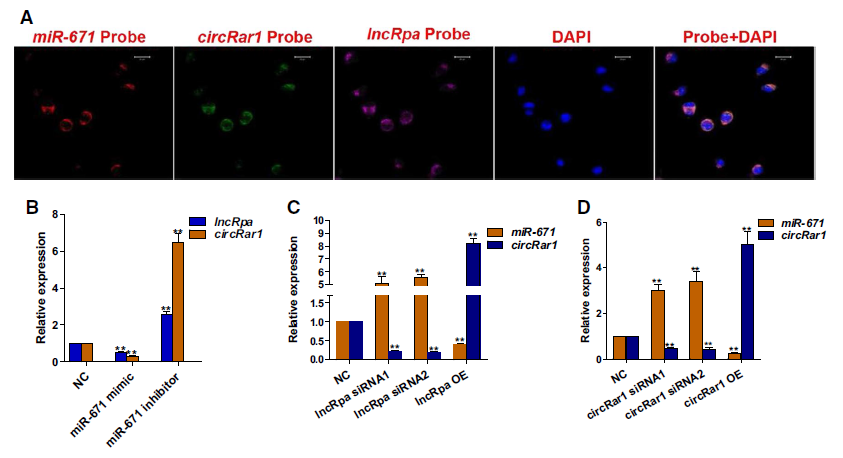
3. LncRpa、circRar1调控miR‑671的表达
荧光原位杂交结果显示lncRpa、circRar1、miR‑671在N2a细胞中共表达。相互之间开展过表达和RNA干扰实验,结果显示lncRpa和circRar1相互促进;miR‑671与lncRpa/circRar1之间则是一种负调控的关系。
伯信合作技术
CircRNA过表达载体构建、荧光原位杂交(FISH)、RNA antisense purification(RAP)
参考文献
Aruo Nan, Lijian Chen, Nan Zhang, et al. A novel regulatory network among LncRpa, CircRar1, MiR-671 and apoptotic genes promotes lead-induced neuronal cell apoptosis[J]. 2016.
原文链接
http://link.springer.com/article/10.1007%2Fs00204-016-1837-1




