摘要:
Background:Malignant glioma is the most lethal primary tumor of the central nervous system, with notable cell invasion causing significant recurrence. Suppression of glioma invasion is very important for improving clinical outcomes. Drugs that directly disrupt the cytoskeleton have been developed for this purpose; however, drug resistance and unsatisfactory selectivity have limited their clinical use. Previously, we reported that protein kinase A (PKA, also known as cyclic-AMP dependent protein kinase) activation induced the differentiation of glioma cells.
Methods:We used several small molecular inhibitors and RNA interference, combined with wound healing assays, Matrigel transwell assay, and microscopic observation, to determine whether activation of the PKA pathway could inhibit the invasion of human glioma cells.
Results:Activation of PKA decreased the invasion of glioma cells. The mechanism operated via transcriptional upregulation of microtubule-associated protein 2 (MAP2), which was activated by the PKA pathway and led to ossification of microtubule dynamics via polymerization of tubulin. This resulted in morphological changes and a reduction in glioma cell invasion. Furthermore, chromosome immunoprecipitation and quantitative real-time polymerase chain reaction showed that signal transducer and activator of transcription 3 (STAT3) is involved in the transcriptional upregulation of MAP2.
Conclusion:Our findings suggested that PKA may represent a potential target for anti-invasion glioma therapy and that the downstream modulators (eg, STAT3/MAP2) partially mediate the effects of PKA.
Keywords: cAMP/PKA pathway, invasion, malignant glioma, MAP2, microtubule.
关键字:cAMP/PKA 途径、侵袭、恶性胶质瘤、MAP2、微管
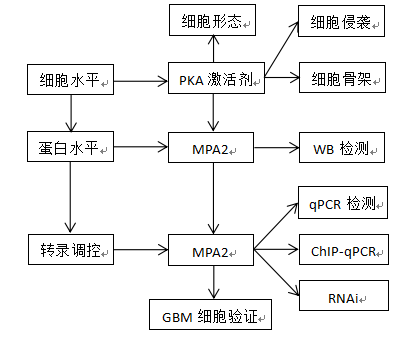
技术流程
研究背景
中枢神经系统恶性胶质瘤是最致命的原发性肿瘤,它具有明显的细胞侵袭性,从而导致肿瘤复发。抑制神经胶质瘤细胞对于提高临床预后至关重要,为了达到抑制肿瘤细胞的目的,研制出了直接破坏细胞骨架的药物。然而,抗药性以及较差的选择性限制了它们的临床应用。在此之前,我们报道过蛋白激酶A(PKA)的活化能够诱导胶质瘤细胞的分化。
研究结果
1、PKA激活剂诱导神经胶质瘤细胞的形态变化,三种神经胶质瘤细胞在3种PKA激活剂的作用下表现出独特的形态变化,细胞变得小而长、更清晰,星型逐渐减少(图1),细胞活性方面与对照表现一致。表明,PKA激活剂能够诱导神经胶质瘤细胞发生明显的形态学变化。
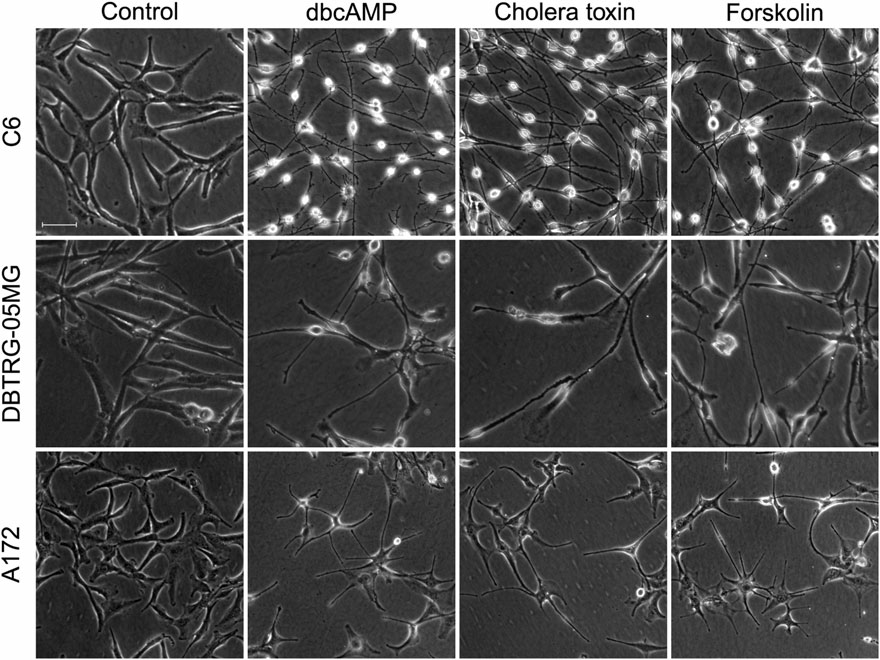
图1 三种神经胶质瘤细胞在3种PKA激活剂作用下的形态变化
注:C6, DBTRG-05MG, and A172 为三种不同的神经胶质瘤细胞,PKA激活剂处理分别为1 mM dbcAMP、10 ng/mL cholera toxin、30 mM forskolin
2、PKA激活剂抑制神经胶质瘤细胞的迁移和侵袭。创伤恢复实验显示,PKA激活剂明显的抑制了三种肿瘤细胞的迁移能力(图2A);侵袭实验中,三种PKA激活剂对C6细胞侵袭的抑制能力超过40%(图2 B、C);结果表明,PKA激活剂能够抑制神经胶质瘤细胞的流动迁移,但不影响其生存能力。
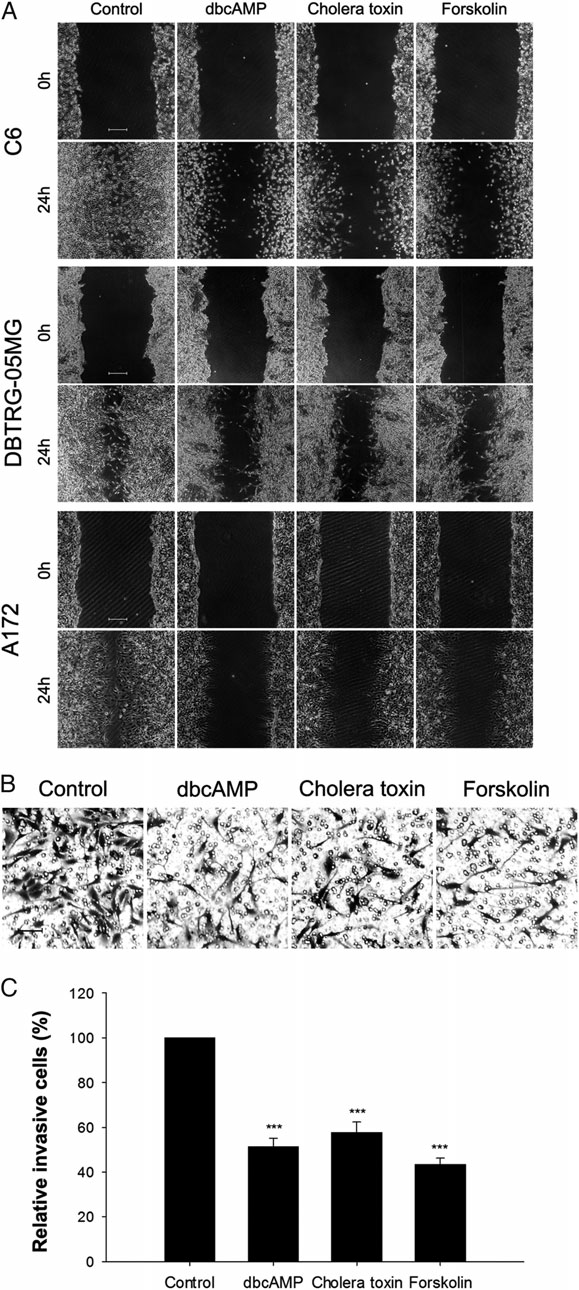
图2 PKA激活剂对神经胶质瘤细胞迁移和侵袭的影响
3、激活PKA能够稳定神经胶质瘤细胞的细胞骨架。PKA激活剂处理后的肿瘤细胞骨架变得更加有序;对比PKA激活剂dbcAMP,PKA抑制剂(KT-5720)处理后,整个细胞被摧毁;进一步显示这种形态学的变化依靠cAMP/PKA途径;Western分析微管的聚合和解离情况,dbcAMP能够提高聚合微管的含量,而KT-5720处理可以抵消这种含量的增加,说明激活cAMP/PKA途径能使微管更加稳定;综上结果表明PKA激活剂引起的微管形态的变化可以神经胶质瘤细胞形态的变化,进一步抑制细胞的迁移和侵袭。
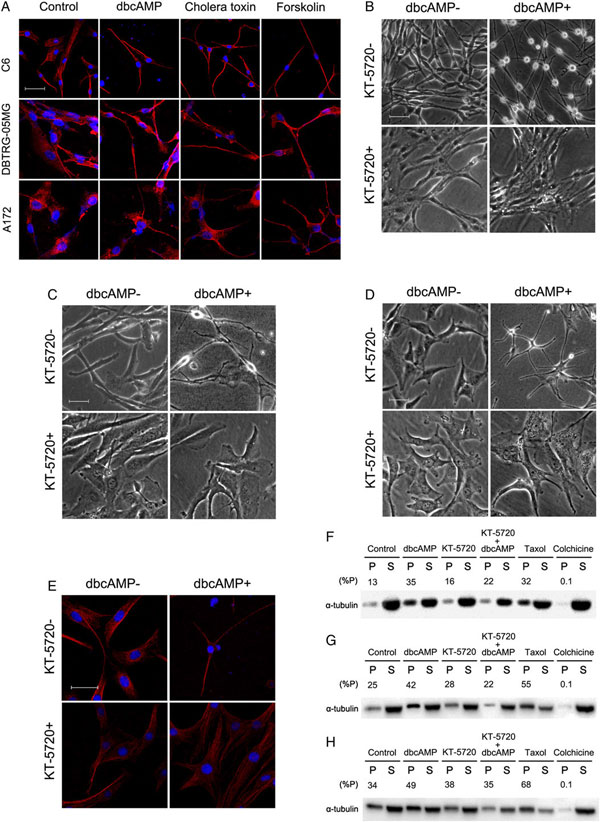
图 3 PKA对细胞骨架的影响
注:dbcAMP为PKA激活剂,KT-5720为PKA抑制剂,C6 (B and E), DBTRG-05MG (C), and A172 (D)
4、PKA激活剂引起MPA2的上调。PKA激活剂处理肿瘤细胞C6,小分子MAP2的蛋白含量显著增加;三种肿瘤细胞的进一步实验中,抑制剂KT5720均能使dbcAMP对MAP2的诱导功能减弱);siRNA干扰PKA的催化亚基(PKAcα),由dbcAMP引起的细胞形态的变化以及MAP2的上调均被解除;综上结果表明,激活cAMP/PKA途径可能通过提高MPA2的表达来影响微管的动力学变化。
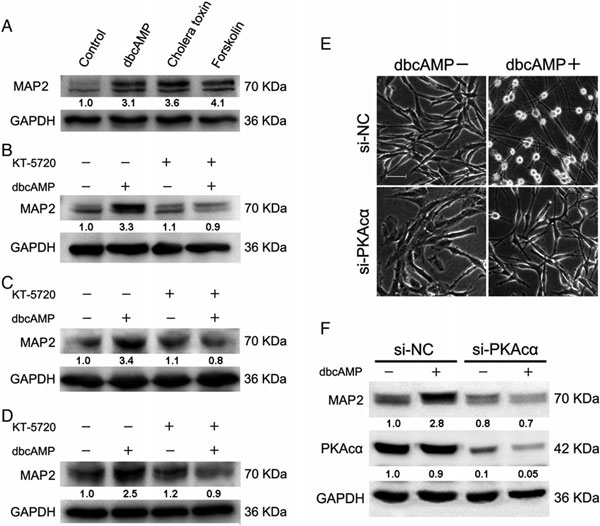
图 4 PKA不同状态对MPA2表达的影响
5、MAP2表达上调参与PKA相关的细胞侵袭性的下降。PCR结果显示PKA激活剂处理能够显著提高MAP2的表达水平,而转录抑制剂则能使这种上调消失(图5A、B);通过UCSC预测MAP2的上游调控序列,发现了STAT3的富集,沉默STAT3能够抑制由dbcAMP引起的MAP2表达上调(图5C),ChIP实验证明STAT3能够与MAP2的启动子区域结合,而且这种结合在dbcAMP处理下得到增强(图5D-);JAK2抑制剂AG490能够降低dbcAMP引起的MAP2的表达上调(图5E);进一步的实验,RNAi引起的MAP2转录损耗抑制了PKA激活剂引起的肿瘤细胞形态学的变化(图5F),这种RNA干扰同样能够解除由dbcAMP引起的肿瘤细胞侵袭的抑制(图5G、H),MAP2转录水平的减少也可以使dbcAMP引起的聚合微管蛋白的增加减缓;综合以上结果,表明MAP2的转录上调能够抑制肿瘤细胞侵袭。
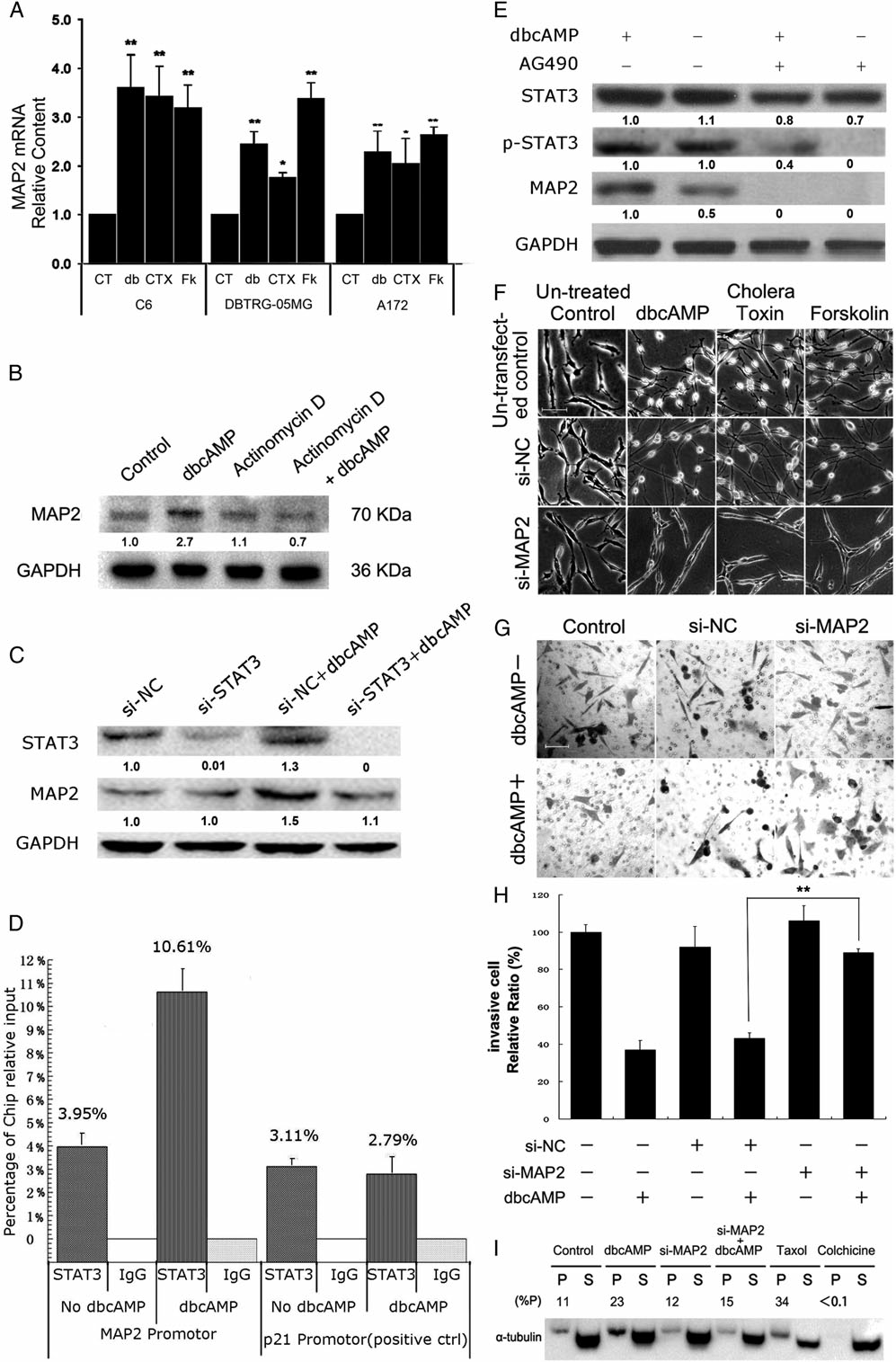
图 5 MAP2表达上调参与PKA相关的细胞侵袭性的下降
6、为了进一步证实PKA激活剂通过诱导MAP2的表达来抑制细胞侵袭,利用原初GBM细胞开展了一系列实验,侵袭实验中,GBM细胞的侵袭能力同样能被dbcAMP抑制(图6A&B),抑制剂KT5720能够解除dbcAMP引起的细胞形态变化(图6C),而且dbcAMP一样可以上调MAP2的表达(图6D-免疫荧光)。说明PKA激活剂确实可以通过诱导MAP2上调来抑制细胞侵袭。
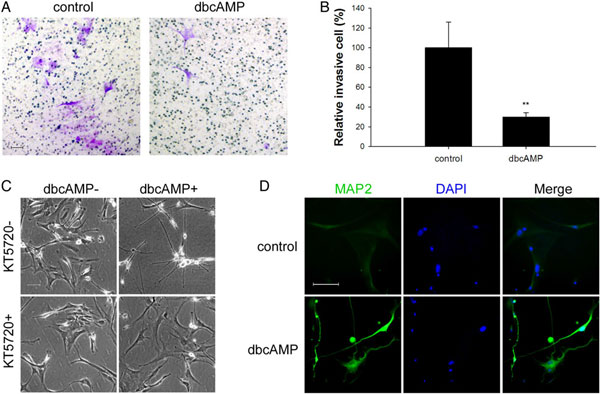
图 6 dbcAMP对GBM细胞的影响
伯信合作技术
ChIP-qPCR、RNAi、western blot、共聚焦检测、细胞侵袭
参考文献
Zhou Y, Wu S, Liang C, et al. Transcriptional upregulation of microtubule-associated protein 2 is involved in the protein kinase A-induced decrease in the invasiveness of glioma cells.[J]. Neuro-oncology, 2015.
原文链接
http://neuro-oncology.oxfordjournals.org/content/early/2015/05/25/neuonc.nov060.full.pdf




