
摘要:
Background: Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)-related tumor. The role of EBV-encoding miR-BART22 is still unclear in NPC. This study aimed to identify the detailed mechanisms by which EBV-miR-BART22 functions as a tumor-promoting factor and evaluate the action of cinobufotalin in treating EBV-miR-BART22-overexpressing NPC cells.
Methods: Using real-time PCR, western blotting, immunohistochemistry, and In situ hybridization, we detected the expression of miR-BART22 and MAP2K4 in tissues and cells, as well as evaluated their clinical relevance in NPC patients. The effects of miR-BART22 on cell metastasis, stemness and DDP chemoresistance were examined by sphere formation assay, side population analysis, transwell, boyden, in vivo xenograft tumor mouse model et al. Western blotting, immunofluorescence staining, luciferase reporter assay, ChIP, EMSA and Co-IP assay et al. were performed to explore the detailed molecular mechanism of EBV-miR-BART22 in NPC. Finally, we estimated the effects and molecular basis of Cinobufotalin on EBV-miR-BART22-overexpressing NPC cells in vitro and in vivo assays.
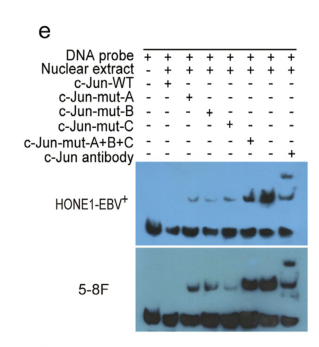
Findings: We observed that EBV-miR-BART22 not only promoted tumor stemness and metastasis, but also enhanced the resistance to Cisplatin (DDP) in vitro and in vivo. Mechanistic analysis indicated that EBV-miR-BART22 directly targeted the MAP2K4 and upregulated non-muscle myosin heavy chain IIA (MYH9) expression by PI3K/AKT/c-Jun-induced transcription. Further, MYH9 interacted with glycogen synthase 3β(GSK3β) protein and induced its ubiquitin degradation by activating PI3K/AKT/c-Jun-induced ubiquitin transcription and the latter combined with increased TRAF6 E3 ligase, which further bound to GSK3β protein. Reductions in the GSK3β protein thus promoted β-catenin expression and nuclear translocation, which induced tumor stemness and the epithelial-to-mesenchymal transition (EMT) signals. Furthermore, we observed that cinobufotalin, a new chemically synthesized compound, significantly suppressed EBV-miR-BART22-induced DDP chemoresistance by upregulating MAP2K4 to suppress MYH9/GSK3β/β-catenin and its downstream tumor stemness and EMT signals in NPC. Finally, clinical data revealed that increased miR-BART22 and reduced MAP2K4 expression caused the poor prognoses of NPC patients.
Keywords: EBV-miR-BART22, Cinobufotalin, DDP chemoresistance, Stemness, NPC
合作部分结果:

伯信合作技术:ISH、EMSA




